Vitamin D and SARS-COV-2
Positioning Paper On Vitamin D and SARS-CoV-2 - Coronavirus – COVID-19, September 11, 2020
Positioning paper on vitamin D and SARS-CoV-2
Coronavirus – COVID-19, September 11, 2020
VITAMIN D AND SARS-COV-2
INTRODUCTION:
Vitamin D (or D3) is a hormone vitamin that after binding to its specific vitamin D receptors known as VDR, plays a role in calcium homeostasis, bones, and a healthy immune system modulation towards fighting respiratory infections.
SARS-CoV-2 is a beta coronavirus and an enveloped virus that causes the COVID19-related respiratory tract infection.
The big question asked about the role of vitamin D in COVID19 is: Does vitamin D3 play a role in COVID-19? And if yes, what role does it play in COV19?
Some of the reasons for looking at vitamin D in COVID19 are because vitamin D deficiency has been associated with an increase in acute respiratory tract infections. Vitamin D has been shown to decrease the risk of multiple respiratory tract infections (RTI) and their severity.
THE EVIDENCE FROM EPIDEMIOLOGY DATA: VITAMIN D AND RESPIRATORY TRACT INFECTIONS:
From historical medical data, we know that vitamin D deficiency is associated with increased susceptibility to a variety of RTI like tuberculosis (TB). It was found that vitamin D actually reduces morbidity associated with TB. In fact, patients with TB used to be hospitalized in sunny sanatoria and clinics. The reason is that, being exposed to the sun would increase the synthesis of vitamin D and help fight off the respiratory bacterial infection.
Another RTI associated with VD deficiency is the common virus of influenza.
The following article: “Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data” is a systematic review and meta-analysis of individual data on the prevention of RTI by vitamin D supplementation. It was published by Martineau Et Al in BMJ 2017.
The meta-analysis shows that vitamin D supplementation is associated with a reduction in acute RTIs.
The figure below highlights the different articles looked at and the adjusted odd ratios. The line represents an adjusted odd ratio of 1, where anything to the right of this line means that there is a positive association between vitamin supplementation and increased RTI. However, anything to the left of this line means that there is a negative association, meaning that vitamin D supplementation decreases RTI. Looking at the combined adjusted ratio, we see that for all of these studies, it comes out to a value of 0.80. Hence, vitamin D supplementation reduces the incidence of RTI by 20% and the data is statistically significant with a p value less than 0.05 and a beautiful confidence interval [CI: 0.69-0.93]
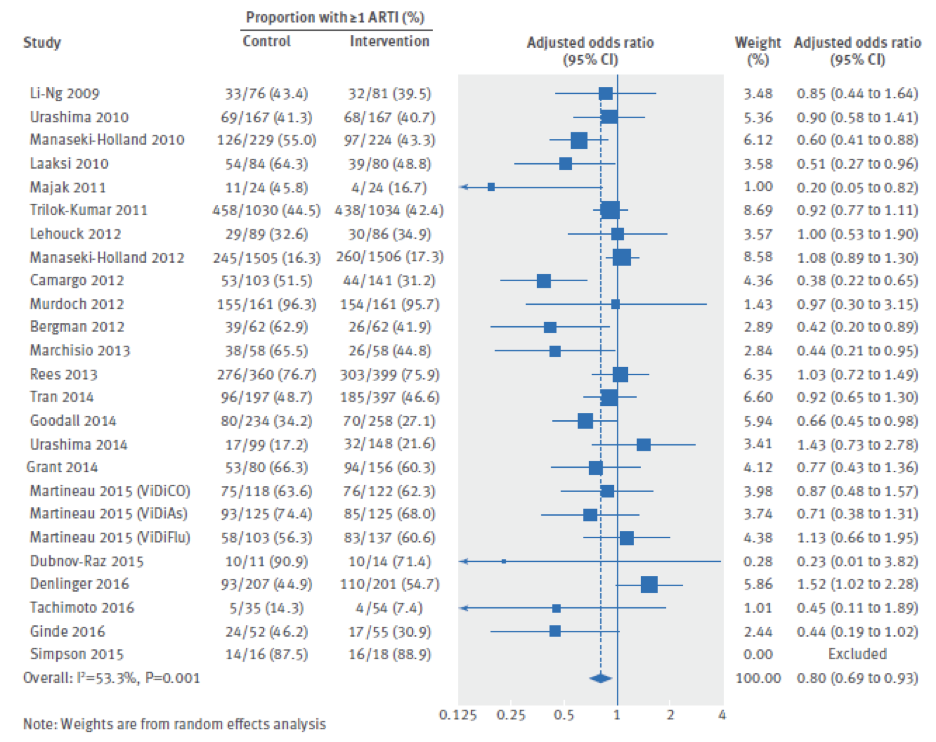
Epidemiological data show that vitamin D supplementation reduces the incidence of RTI by 20%.
Also, the authors of this meta-analysis did a subgroup analysis where they looked more specifically at certain groups of participants. It was found that vitamin D supplementation has the strongest protective effects in groups with vitamin D deficiency, mainly the group where vitamin D levels were less than 10 ng/ml (or 25nmol/L).
The authors also looked at how dosing plays a role in vitamin D supplementation and acute RTI. What they found was that dosing is also an important factor. Daily or weekly dosing of vitamin D was found to be protective. But bolus dosing did not show a protective effect. Here, bolus represents a very large amount of VD taken all at once and not divided over a period of time.
Taking vitamin D over a long time at appropriate doses, like those recommended by the Institute of Medicine (IOM), are better at reducing the risk of RTI. A typical recommended daily dose ranges between 1000-4000 IU.
As per the IOM, 4000 IU represents the upper limit for daily dosing. To be on the safe side, and for an effective regimen, a dose of 3000 IU daily would be ideal.
Very recently, and in a population of non-white Americans*, the relation between vitamin D levels and contracting coronavirus was evaluated. Indeed, it was shown that among the group who had plenty of vitamin D, only 12 percent (12%) tested positive for coronavirus. However, and remarkably, nearly 20 percent (19%) of those who had low levels of vitamin D tested positive. It’s hardly proof that vitamin D can keep people from catching coronavirus, but the 60 percent difference in positive test rates suggests it may have some effect.
In dark skin people (black or brown), higher levels of melanin in the skin make it more difficult for their bodies to absorb the vitamin.
There are 60% higher rates of COVID-19 among people with low levels of vitamin D compared to people with normal levels.
In an Intensive Care Unit (ICU) setting, only two percent (2%) of the patients who received a high dose of vitamin D in addition to a combination of hydroxychloroquine and azithromycin were admitted to ICU compared to 50% of those who only received hydroxychloroquine and azithromycin. This represents a 96% reduction of risk of admission to ICU or when expressed as a relative risk, vitamin D reduced the risk of ICU admission 25-fold (p<0.001, 95% CI [0.002-0.17]).
However, the levels of vitamin D were not measured and based on mathematical models. The large bolus and weekly doses used later in those patients would presume that the patients’ vitamin D levels were brought into the 30-40 ng/mL range by the end of the first week, and that most of the healing took place around the 40 ng/mL range and that by the time the patients were discharged from the hospital, their vitamin D levels would have exceeded 50 ng/mL.
There was no mortality in the group that received vitamin D vs. 2 deaths occurring in the group that only received hydroxychloroquine and azithromycin.
Regardless of the large bolus dose given twice in these hospitalized patients, a reasonable approach to mimic the 7000 IU taken daily would be to encourage people in need to consume vitamin 3000 IU BID or 50,000 IU weekly.
This represents a 96% reduction of risk of admission to ICU.
Vitamin D reduced the relative risk for ICU admission by 25-fold.
3000 IU BID vitamin D (6000 IU daily) is close enough to 50,000 IU weekly.
TARGET LEVEL FOR VITAMIN D IN THE BLOOD:
Data from many observational studies that were either published or remained as preprints during the pandemic period, converged on the common point that 30-40 ng/mL seems to the be the target levels where infection risk, severity, and mortality are all the lowest, without any risk of getting side-effects from too much vitamin D. However, some data predict a near abolition of risk of infection with plasma vitamin D levels > 53 ng/mL, as per the article “Low plasma 25(OH) vitamin D level is associated with increased risk of COVID -19 infection” published in July 2020 in FEBS journal by Merzon E et al.
Target a 30 – 40 ng/ml levels of vitamin D in blood or slightly higher.
MECHANISM OF ACTION: PLAUSIBLE CAUSES:
After reviewing the epidemiological data and the increased correlation between vitamin D deficiency and RTIs, the question to be asked now is: How does vitamin D actually protect against RTI?
Because vitamin D receptors are actually present in many immunological cells like the dendritic cells, B cells, T cells and macrophages, it is evident that vitamin D is involved in immune system regulation.
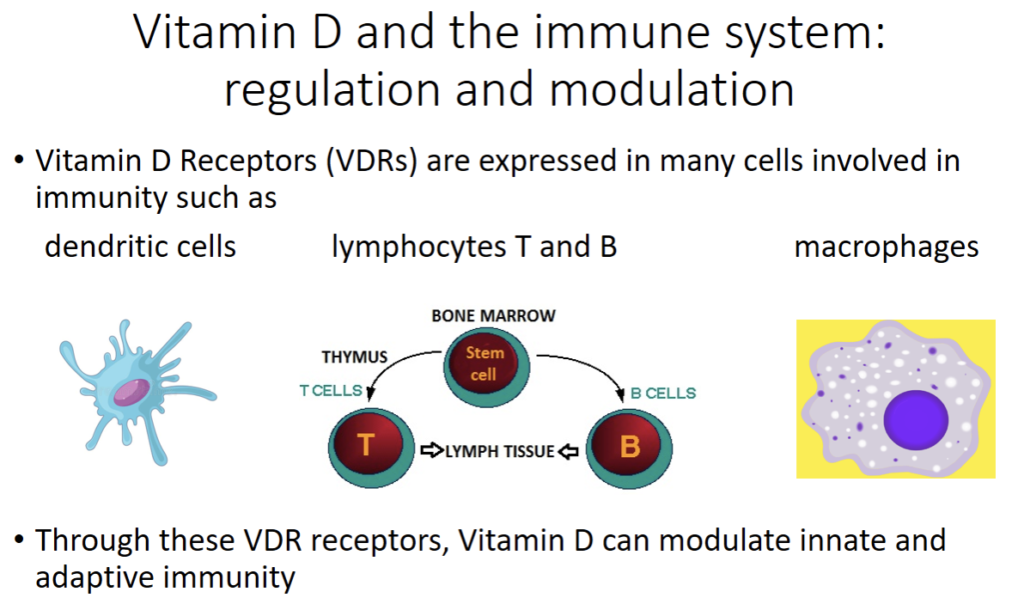
Vitamin D has been shown to regulate the expression and production of many proteins involved in immunity. In fact, by way of activating their vitamin D receptors (VDRs), vitamin D can modulate innate and adaptive immune system function with the upregulation of immune system proteins like cathelecidins and defensins. These antimicrobial proteins have antibacterial, antifungal and antiviral properties.
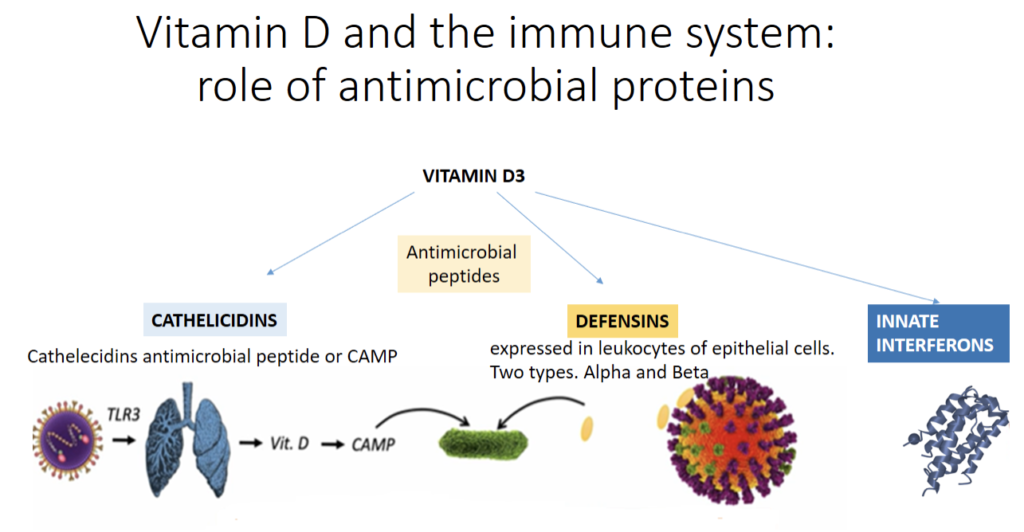
The figure above describes some of these mechanisms. Vitamin D upregulates cathelecidins and defensins. This mechanism is complex.
Cathelecidins Antimicrobial Peptide or CAMP is activated when viruses responsible for RTI come into contact with respiratory epithelial cells. Viruses activate these cells through toll-like receptors 3 (TLR3) that are pattern recognition receptors of the innate immune system. This leads to the activation of the vitamin D pathway leading to induction of CAMP.
Cathelicidins exhibit direct antimicrobial activities against a spectrum of microbes, including gram-positive and gram-negative bacteria, enveloped viruses, and fungi. It is of value to remember that corona and influenza viruses are enveloped viruses.
For defensins, they are expressed in leukocytes of epithelial cells. Two types of defensins exist: Alpha (α) and Beta (β). Defensins bind to the influenza virus and cause aggregation of the virus and reduce its ability to infect cells.
Cathelicidins, defensins and innate interferon are all involved in the chemo-attraction of various immune cells that help recognize the invading virus.
Cathelicidins and defensins:
- Disrupt microbial membranes
- Reduce viral replication
- Reduce the occurrence of cytokine storm by shifting the inflammatory response from a pro-inflammatory response to an anti-inflammatory one.
By binding to the VDRs, vitamin D can modulate innate and adaptive immune system function
Vitamin D upregulates cathelecidins and defensins.
Cathelicidins, defensins and innate interferon are all involved in the chemo-attraction of various immune cells that help recognize the invading virus.
This decreases viral replication.
CYTOKINE STORM:
The cytokine storm is related to Acute Respiratory Distress Syndrome (ARDS), that is a critical component in mortality from COVID19. Vitamin D reduces cytokine storm (or syndrome) that causes an increase in morbidity and mortality in RTI and particularly COVID 19. Vitamin D reduces the production of the pro-inflammatory cytokines involved in the cytokine storm such as, Tumor Necrosis Factor alpha (TNFα), interferon gamma (IFNγ) and interleukin-6 (IL-6). Increased levels of IL-6 are indicative of the severity of COVID19, including mortality from COVID19.
Cytokine storm causes an increase in morbidity and mortality in RTI and particularly, in COVID-19.
Vitamin D reduces cytokine storm and IL-6, thus, reducing morbidity and mortality.
THE CORRELATION BETWEEN VITAMIN D DEFICIENCY AND CASE FATALITY RATES:
Case Fatality Rate (CFR), sometimes called case fatality risk or case-fatality ratio, is in epidemiology, the proportion of deaths from a certain disease compared to the total number of people diagnosed with the disease for a particular period.
The article by Grant W et al: “Evidence that Vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths” published in April in Nutrients, looked at the evidence that vitamin D supplementation could reduce the risk of severe infection and death from influenza and COVID-19.
What is currently known, is that the case fatality rate (CFR) incidence in COVID-19 infection is increased in patients with comorbidities like diabetes, hypertension, heart failure, chronic obstructive pulmonary disease (COPD), etc. Indeed, these patients are at an increased risk of being infected with higher morbidity or mortality. Also, the incidence of CFR from COVID-19 is increased in areas of high levels of pollution, in infection with complications like ARDS, in older patients, in patients with high CRP levels or sepsis and high levels of IL-6 as described earlier. What’s interesting is that most of these complications and comorbidities have an inverse relationship or inverse correlation to vitamin D levels.
A plausible explanation of this observation will be stated further below.
Vitamin D levels are inversely correlated with many morbidity factors.
Comorbidities and complications from COVID-19 and vitamin D levels go in opposite directions.
Below is a table showing how vitamin D is related to the clinical and epidemiological findings for incidence of diseases, health conditions and case-fatality rates:
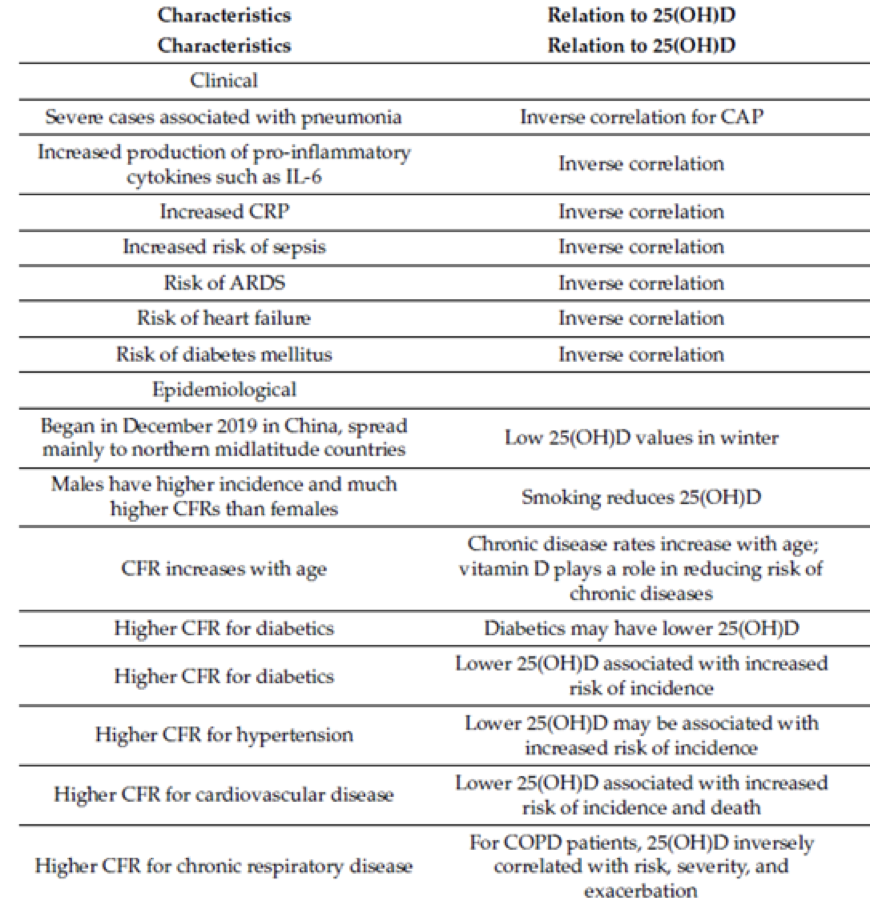
Note: 25-hydroxyvitamin D ((25(OH)D); acute respiratory distress syndrome (ARDS); community-acquired pneumonia (CAP); case-fatality rate (CFR); interleukin 6 (IL-6); chronic obstructive pulmonary disease (COPD); C-reactive protein (CRP); vitamin D deficiency (VDD).
VITAMIN D AND SARS-COV-2 INFECTIVITY – THE ROLE OF THE ACE2 RECEPTORS AND THE RENIN-ANGIOTENSIN-ALDOESTERONE SYSTEM (RAAS)
What we know so far is that the clinical response to COVID-19 infection possibly depends on 3 dominant factors:
- The initial viral load
- The integrity of the immune system
- These two factors are very important but the third factor is no less of importance than the first two and this is the number and configuration of the surface ACE2 metalloprotein that serves as a port of entry for the viral particles

VITAMIN D AND SARS-COV-2 INFECTIVITY – THE ROLE OF THE ACE2 RECEPTORS AND THE RENIN-ANGIOTENSIN-ALDOESTERONE SYSTEM (RAAS)
Angiotensin II (Ang II) is a natural peptide hormone in the renin-angiotensin-aldosterone system. Ang II increases blood pressure through direct systemic vasoconstriction and by stimulating aldosterone. ACE2 has a beneficial effect of directly catalyzing Ang II, thereby lowering its levels. Lowering levels of Ang II would improve associated cardiovascular and cardiometabolic morbidities. High levels of Ang II may cause ARDS, myocarditis or cardiac injury. On the other hand, when ACE2 receptors are functioning in a proper way – cleaved and activated – they would interact with Ang II transforming it to Angiotensin 1-7 (Ang [1-7]), that has no harmful metabolic effects on health. In fact, Ang [1-7] lowers blood pressure and appears to have vasodilatory, anti-inflammatory and antioxidant effects, reducing inflammation and atherosclerotic disease.
ACE2 receptor is a transmembrane zinc metalloprotein found in blood vessels, respiratory epithelium, gastro-intestinal tract and kidney vessels. SARS-COV2 seeks out this specific ACE2 surface protein molecular target to gain access inside of our cells. This explains the symptoms in COVID19 like respiratory symptoms, kidney failure, dementia etc. Indeed, data from previous coronavirus infections like the SARS-COV-1 strain showed that ACE2 protein treatment inhibited the spreading of SARS-COV-1 and also protect patients with SARS-COV-1 infection from developing lung failure. Moreover, a single dose of recombinant human ACE2 treatment prevents pulmonary arterial hypertension both in clinical and preclinical studies.
So, after binding to ACE2 receptors and invading the host cells, COVID-19 infection will downregulate ACE2, which in return could initiate excessive accumulation of Ang II leading to all the harmful effects of increased RAAS stimulation.
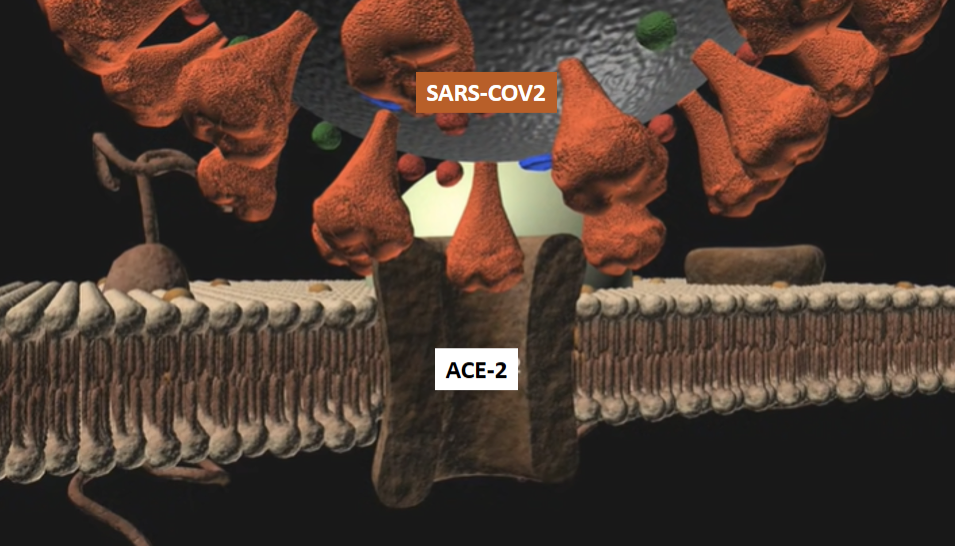
The above picture illustrates the coronavirus attaching to ACE2 receptors on lung epithelia cells prior to invading the body of the infected host. This is followed by the downregulation of the ACE2 receptors and increased Ang II production.
Following the SARS-COV2 infection, the spectrum of COVID-19 clinical response between individuals ranges from asymptomatic infection to hospitalization, need for assisted ventilation or a respirator and possibly death. Although many factors are incriminated in the difference and severity of clinical response between individuals, nonetheless, this could partly be due to the possible person to person variability in either the configuration or the number of ACE2 transmural proteins, mainly in the pulmonary epithelium.
Indeed, as reported above, patients with co-morbidities like diabetes, cardiovascular disease, obesity, and other diseases, were found to be more predisposed to infection and have it more severely. In those patients, the balance between Ang II and Ang [1-7] is tipped in favor of Ang II due to the activation of the RAAS. This would downregulate the ACE2 receptors and allow for the SARS-COV-2 to attach through its spike proteins to the pulmonary ACE2 receptors and invade the host cells, leading to further downregulation of the ACE2 receptors. An angiotensin balance that is shifted more towards Ang [1-7] like in healthy individuals, would translate into more abundant ACE2 receptors with some kind of resistance being conferred to those receptors from being cleaved by some proteases, obstructing infectivity by the virus.
A new study showed that human recombinant soluble ACE2 treatment may significantly suppress early stages of SARS-CoV-2 infections.
Vitamin D deficiency leads to less ACE2 activation, hence, more production of harmful Ang II and less production of the beneficial Ang [1-7].
Vitamin D upregulates ACE2 receptors.
COVID-19 infection downregulates ACE2.
VITAMIN D – SUMMARY OF CLINICAL AND IMMUNOLOGICAL BENEFITS:

A) Vitamin D might prevent the development of ARDS, hypertension and cardiovascular system disorders by reducing the level of renin, ACE and angiotensin II and increasing the level of ACE2.
B) Vitamin D can prevent the development of oxidative stress and organ damage by reducing inflammatory cytokines in many tissues and preventing the cytokine storm from occurring.
C) Vitamin D may enhance antimicrobial activity by increasing defensins and cathelicidins peptides level. This could be effective against the COVID-19 infection.
CONCLUSIONS:
While a lot about the role of vitamin D in strengthening innate immunity of individuals in RTI and especially in COVID-19 pandemic has been revealed, a lot more needs to me elucidated on what we think is already known about the mechanism or action, dosage, target population (blacks and Hispanics) and potential treatment of COVID-19.
Vitamin D deficiency is a consequence of chronic health conditions or behavioral factors that could increase COVID-19 risk. Chronic health conditions were clearly associated with higher infectivity cases and increased morbidity and mortality associated to COVID-19 infection (increased case fatality rates).
In order to remove any doubt about the importance of vitamin D in COVID-19 pandemic, interventions to reduce vitamin D deficiency, as a mean to reduce COVID-19 risk, should be tested in randomized clinical trials.
If vitamin D does reduce COVID-19 incidence, it is tempting to consider whether it might also reduce COVID-19 transmission, hence, the need of clinical studies on that endpoint.
About the dose, and since most data agree on the fact that vitamin D deficiency leads to increased infectivity and seriousness of the COVID-19 disease, the aim of vitamin D supplementation should therefore be to bring individuals to normal vitamin D levels and preferably to the range of 30-40ng/ml. Any dose can be used (with the exception of high bolus doses that is reserved to hospital setting) to achieve this goal.
Daily doses of 1000 – 4000 IU have been shown to ensure adequate levels of vitamin D in a very safe way.
References:
- Rathish Nair and Arun Maseeh. Vitamin D: The “sunshine” vitamin. J Pharmacol Pharmacother. 2012 Apr-Jun; 3(2): 118–126
- Grant WB et al. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988; doi:10.3390/nu12040988
- Martineau AR and Forouhi NG. Vitamin D for COVID-19: a case to answer? thelancet.com/diabetes-endocrinology Vol 8 September 2020.
- Hatice Aygun. Vitamin D can prevent COVID-19 infection-induced multiple organ damage. Naunyn Schmiedebergs Arch Pharmacol. 2020 May 25: 1–4
- Castillo ME et al. Effect of Calcifediol Treatment and best Available Therapy versus best Available Therapy on Intensive Care Unit Admission and Mortality Among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical study. The Journal of Steroid Biochemistry and Molecular Biology. Available online 29 August 2020, 105751. Journal Pre-proof.
- Merzon E et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID‐19 infection: an Israeli population‐based study. Federation of European Biochemical Societies (FEBS) Journal. 2020 Jul 23: 10.1111/febs.15495.doi: 10.1111/febs.15495
- Heurich A et al. TMPRSS2 and ADAM17 Cleave ACE2 Differentially and Only Proteolysis by TMPRSS2 Augments Entry Driven by the Severe Acute Respiratory Syndrome Coronavirus Spike Protein. Journal of Virology; 2014,88(2):1293–1307
- Samavati L and Uhal BD. ACE2, Much More Than Just a Receptor for SARS-COV-2. Front. Cell. Infect. Microbiol., 05 June 2020, 10 (317)
- Wiese O et al. Molecules in pathogenesis: angiotensin converting enzyme 2 (ACE2). J Clin Pathol 2020;0:1–6
- Hasan A et al. A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin. JOURNAL OF BIOMOLECULAR STRUCTURE AND DYNAMICS. doi.org/10.1080/07391102.2020.1754293
- Kuba K et al. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2.
- J Mol Med (Berl). 2006 Oct; 84(10):814-20
- Monteil V et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020; S0092-8674(20):30399–30398
- Hemnes AR et al. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur Respir J. 2018 Jun; 51(6).
- Meltzer DO et al. Association of Vitamin D Status and Other Clinical Characteristics with COVID-19 Test Results. JAMA Network Open. 2020;3(9): e2019722. doi:10.1001/jamanetworkopen.2020.19722